most negative electron affinity|why does electron affinity decrease down : Cebu The electron affinity of molecules is a complicated function of their electronic structure. For instance the electron affinity for benzene is negative, as is that of naphthalene, while those of anthracene, phenanthrene and pyrene are positive. In silico experiments show that the electron affinity of hexacyanobenzene surpasses that of fullerene. Watch Pinay Ofw Kinantot Ng Amo porn videos for free, here on Pornhub.com. Discover the growing collection of high quality Most Relevant XXX movies and clips. . Chubby Pinay Katulong Kinantot ng Amo Habang Naglilinis Maid Fuck while Cleaning - Pinay Viral 2024 . PINAY KASAMBAHAY NA NAG LILINIS NG REF KINANTOT NG AMO NIYA-PINAY .
most negative electron affinity,Ago 11, 2023

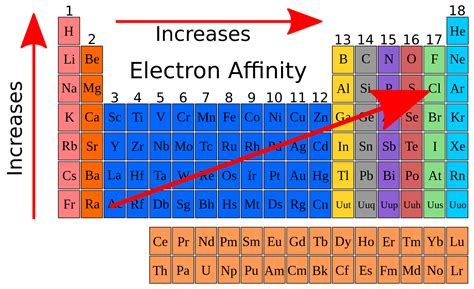
The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom . Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 16 and 17 of the Periodic Table. The first . The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. Electron affinity can be .The electron affinity of molecules is a complicated function of their electronic structure. For instance the electron affinity for benzene is negative, as is that of naphthalene, while those of anthracene, phenanthrene and pyrene are positive. In silico experiments show that the electron affinity of hexacyanobenzene surpasses that of fullerene. Negative electron affinities can be used in those cases where electron capture requires energy, i.e. when capture can occur only if the impinging electron has a kinetic . About. Transcript. Electronegativity is a measure of an atom's ability to attract shared electrons to itself. On the periodic table, electronegativity generally increases as you move from left .
Learn what electron affinity is, how it is measured and what factors affect its size. Compare the electron affinities of group 6 and 7 elements and see why fluorine is an .

Electron affinity is related to electronegativity of elements. Simply speaking, the greater the affinity of electrons, the more eagerly the atoms of a given element join .The elements with the highest ionization energies are generally those with the most negative electron affinities, which are located toward the upper right corner of the . 8.8: Electron Affinities and Metallic Character is shared under a license and was authored, remixed, and/or curated by LibreTexts. The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron affinities ..The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom: E(g) +e− → E−(g) energy change=EA (2.8.1) (2.8.1) E ( g) + e − → E ( g) − .
The electronic affinity is amount of energy, that is released during the attachment of the electron to the neutral atom. As a result of such attachment, a negative ion (anion) is formed. Electron affinity is related to electronegativity of elements.Simply speaking, the greater the affinity of electrons, the more eagerly the atoms of a given element join .
Electron Affinity. In most cases, the formation of an anion by the addition of an electron to a neutral atom releases energy. . Electron affinities are negative numbers because energy is released. (Credit: Christopher Auyeung; Source: CK-12 Foundation; License: CC BY-NC 3.0(opens in new window)) The elements of the halogen group . The change in energy ΔE has a negative sign and the electron affinity E ea has a positive sign. When atoms absorb energy, the reaction is endothermic. The change in energy ΔE has a positive sign and the electron affinity E ea has a negative sign. Electron affinity for most atoms on the periodic table, except the noble gases, is .why does electron affinity decrease downThe electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E(g) +e− → E−(g) energy change=EA (8.4.1) (8.4.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is .The electron affinity [EA] is the energy change for the process of adding an electron to a gaseous atom to form an anion (negative ion). X(g) +e− X−(g) EA1 (3.4.1) (3.4.1) X ( g) + e − X − ( g) EA 1. This process can be either endothermic or exothermic, depending on the element. The EA of some of the elements is given in Figure 3.4.6 3.4.The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favourable the electron addition process is. Not all elements form stable negative ions in which case the electron affinity is zero or even positive.
The equations for second and higher electron affinities are analogous to those for second and higher ionization energies: E ( g) + e − → E − ( g) energy change=EA1. E − ( g) + e − → E2 − ( g) energy change=EA2. As we have seen, the first electron affinity can be greater than or equal to zero or negative, depending on the .Rank the following elements by electron affinity, from most positive to most negative EA value: Tellurium, Bismuth, Neon, Sodium, Iodine. Arrange the members of the Group 4A metals in order of increasingly negative electron affinities. 8.8: Electron Affinities and Metallic Character is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron .most negative electron affinity why does electron affinity decrease downThe electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom: E(g) +e− → E−(g) energy change=EA (2.10.1) (2.10.1) E ( g) + e − → E ( g) − . The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E(g) +e− → E−(g) energy change=EA (8.5.1) (8.5.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is .The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E(g) +e− → E−(g) energy change=EA (7.5.1) (7.5.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is . Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an electron to form F⁻(g), the .
Of the following elements, _____ has the most negative electron affinity. A) Na B) Li C) Be D) N E) F. Like. 0. All replies. Answer. 1 year ago. The correct answer is option E. Electron affinity is defined as the tendency of an atom to accept electrons. The atoms having negative electron affinity readily accept theChemists define electron affinity as the change in energy, measured in units of kJ/mole, experienced when an electron is added to a gaseous atom. This process creates a negative ion. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. We tend to liken electron affinity to .Summary. The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right .
most negative electron affinity|why does electron affinity decrease down
PH0 · why does electron affinity decrease down
PH1 · ionic or covalent calculator
PH2 · how does electronegativity differ from electron affinity
PH3 · greatest electron affinity
PH4 · electronegativity chart
PH5 · electron affinity trend and exceptions
PH6 · difference between electronegativity and electron affinity
PH7 · arrange these elements according to electron affinity
PH8 · Iba pa